Watch this video to dig a little deeper into the history and wave-particle duality of light.
Ask yourself: Can you explain how light acts like both a particle and a wave?
Hi, I’m Rachel, the Astronomer here at the Space Centre.
On Earth, our main source of light is the Sun. Solar radiation, or sunlight, provides the energy for photosynthesis – plants create sugars, which when digested, release energy. Some species of animals, like fireflies, can even generate their own light through bioluminescence. In physics, “light” refers to any wavelength of electromagnetic (EM) radiation – from radio waves and microwaves to x-rays and gamma rays. The light that we can see, visible light, is only a portion of the electromagnetic spectrum.
But the history of trying to understand light is a long and confusing one; some scientists proposed that light behaved like a shower of particles, with physicist JJ Thomson’s 1897 discovery of the electron providing more evidence of the particle-like nature of light. Other scientists demonstrated that light behaved like waves, much like waves of water in the sea. In short, it looked like light could be a particle or a wave – so which is it? This very question gave rise to the concept of wave-particle duality.
In 1938, Einstein wrote, “It seems as though we must sometimes use the one theory and sometimes the other, while at times we may use either… We have two contradictory pictures of reality; separately neither of them fully explains the phenomena of light, but together they do.”
This conundrum was resolved in the mid-1920s with the advent of the correct equations for quantum mechanics. Wave-particle duality is deeply embedded in quantum theory. Without diving into the complex mathematics of the quantum world, we can still paint a rough picture of the breakthroughs from physicists Erwin Schrödinger, Werner Heisenberg, Max Born and others, that paved the way for modern quantum mechanics and later, quantum field theory.
But back to this apparent paradox about light or EM radiation behaving like a wave and a particle. Let’s try a short thought experiment (or as Einstein would say, a gedanken-experiment). Say we shine a laser beam on a plate with two parallel slits – as Thomas Young did in 1801 in his famed double-slit experiment – and see what comes out on a screen behind the plate. The wave nature of light causes the light waves passing through the two slits to interfere, producing a fringe pattern of bright and dark bands on the screen.
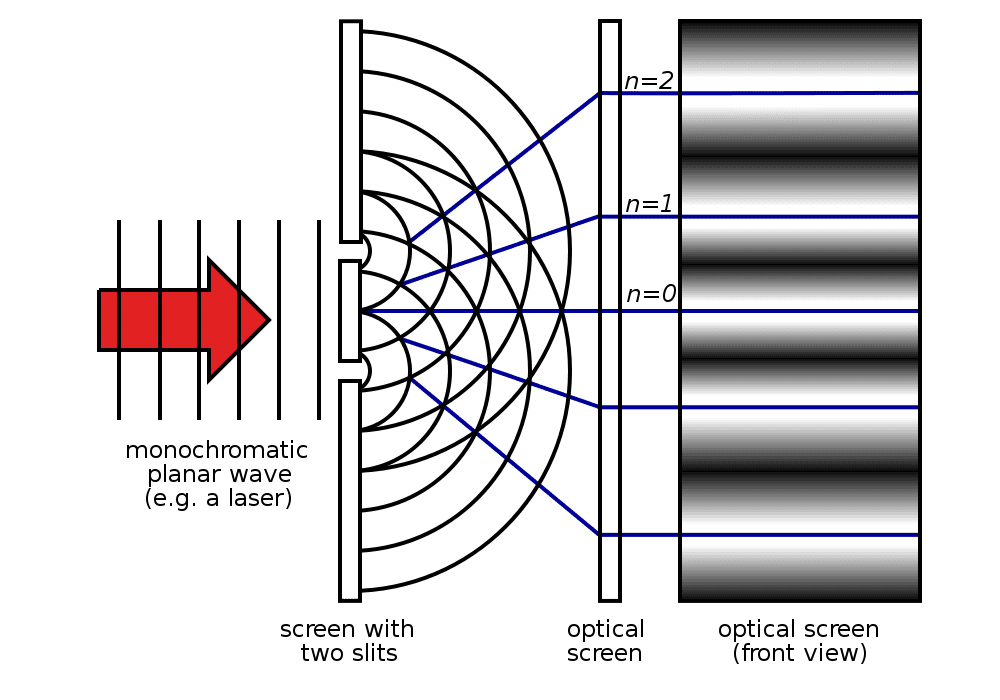
However, if we look at the screen closely, we’ll find that the energy imparted by the waves is absorbed at single locations (the way particles are absorbed). (To see what the particles impacts would look like have a look at this gif.)
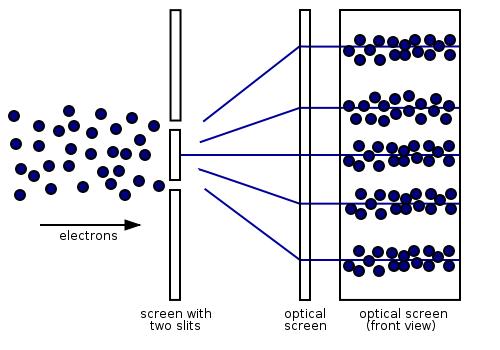
So, what’s going on here?
Herein lies the genius of quantum theory; but first, some basics. A mathematical function, called the wave function, provides information (the probability amplitude) on the energy, momentum, and other physical properties of a particle. When energy from EM waves is absorbed (like when it hits the screen), it is called a photon and represents a quanta, or packet, of light. When a wave of light is absorbed, the energy of the wave now collapses instantly to a single location when measured. This location is where the photon “arrives”. Physicists call this the wave function collapse and is the solution to the dual wave-like and particle-like nature of light
If you’re still a bit confused, don’t worry! It took physicists a number of years to figure this out. I always say that the best way to learn is by doing, so try out Young’s double-slit experiment for yourself. We’ve also compiled a wealth of resources for you below to further explore the mysteries of light.
5 mins
Watch this video to dig a little deeper into the history and wave-particle duality of light.
Ask yourself: Can you explain how light acts like both a particle and a wave?
15 mins
Discover the secret colours hidden in a black marker and think about the role light plays in helping us see colours.
Ask yourself: Why does black ink look black?
15 mins
Using your knowledge about colours, think about what happens to light from the Sun as it travels through Earth’s atmosphere. Then read this article to find out why the sky looks blue!
Ask yourself: Based on what you learned from the article can you explain why sunsets are orange?
30 mins
Impress someone with an optical illusion that depends on the physics of refraction, then try explaining to them how the illusion works.
Ask yourself: Why does the arrow appear to change direction when you pour the water into the cup?
10 mins
Learn about the different types of optical telescopes.
Ask yourself: What type of telescope would you rather have, a refractor telescope or a reflector telescope?
15 mins
Explore the electromagnetic spectrum and discover more about the different types of telescopes that can see different kinds of light.
Ask yourself: Do all kinds of light travel at the same speed?
5 mins
Watch this video to dig a little deeper into the history and wave-particle duality of light.
Ask yourself: Can you explain how light acts like both a particle and a wave?
15 mins
Watch this animation to help you visualize the wave-particle duality of light, then check your understanding with a short quiz.
Ask yourself: What does the presence of an observer change about the diffraction pattern?
10 mins
Discover more about the properties of the electromagnetic spectrum by finding out how energy relates to wavelength and how scientists use spectroscopy to analyze these wavelengths.
Ask yourself: Why is it useful to study an object’s emission spectra?
10 mins
Discover the beauty of multiwavelength astronomy which can be used to image objects like the Crab Nebula.
Ask yourself: Why does the Crab Nebula “shine” in so many different
wavelengths?
120+ mins
Build a papercraft spectrometer for your phone, then use it to analyze the emission spectra of a gas light source or of sunlight.
Ask yourself: Can you think of some other experiments you can do with a spectrometer?